| CAS
NO. |
6132-04-3 |
|
| EINECS NO. |
200-675-3 |
| FORMULA |
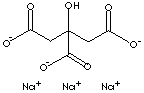
HOC(COONa)(CH2COONa)2.2H2O |
| MOL
WT. |
294.10 |
|
H.S.
CODE
|
2916.31 |
|
TOXICITY
|
|
| SYNONYMS |
Sodium Citrate Dihydrate;
|
| 2-Hydroxy-1,2,3-propanetricarboxylic acid, trisodium salt, dihydrate;
|
| SMILES |
|
|
CLASSIFICATION
|
|
|
PHYSICAL AND CHEMICAL PROPERTIES
|
| PHYSICAL
STATE |
Odourless, white solid in various forms |
| MELTING POINT |
150
C |
| BOILING
POINT |
Decomposes |
| SPECIFIC GRAVITY |
1.665 |
| SOLUBILITY
IN WATER |
71gr/100ml at 25
C (but insoluble in ethanol) |
| PH |
7.0-9.0 (5% solution) |
| VAPOR DENSITY |
|
| AUTOIGNITION |
|
| NFPA RATINGS |
Health: 1 Flammability: 0 Reactivity: 0 |
|
REFACTIVE
INDEX
|
|
| FLASH
POINT |
|
| STABILITY |
Stable
under ordinary conditions but caking may occur on prolonged storage |
|
APPLICATIONS
|
|
pH buffering,
anticoagulant agent, flavor enhancer and
modifier, promotes
sucrose inversion, stabilizer of emulsified fats in the food, beverage and
pharmaceutical industries. also used as a bio-degradable builder in detergents.
|
| SALES
SPECIFICATION |
|
BIBLIOGRAPHY
|
FCC IV/ USP24 /
BP93 |
|
IDENTIFICATION |
Passes
test
|
|
APPEARANCE
|
white
granular crystals or white crystalline powder |
|
ASSAY |
99.0-100.5 % (180
C 18
Hours) |
|
LOSS ON DRYING |
10.0 - 13.0% |
|
ALKALINITY |
Passes
test
|
|
TARTARATE |
Passes
test
|
|
OXALATES |
100ppm max |
|
CHLORIDE |
50ppm
max
|
|
SULFATES |
150ppm
max
|
|
ARSENIC |
1ppm
max
|
|
HEAVY METALS |
5ppm
max
|
|
LEAD |
0.5ppm
max
|
|
IRON |
5ppm
max
|
|
SOLUTION CLARITY |
98
min (%T in
420 nm, 40% Sol.) |
| TRANSPORTATION |
| PACKING |
25kgs
in Bag |
| HAZARD CLASS |
Not
regulated |
| UN
NO. |
|
| GENERAL
DESCRIPTION OF CITRIC ACID
|
|
Citric Acid (2-Hydroxy-1,2,3-propanetricarboxylic acid, in IUPAC naming) is a
colourless crystalline organic compound belong to carboxylic acid family. It
exists in all plants (especially in lemons and limes) and in many animal tissues
and fluids. In biochemistry, it is involved in important metabolism of almost
all living things; the Krebs cycle (also called citric acid cycle or
tricarboxylic acid cycle), a part of the process by which animals convert food
to energy. Citric acid works as a preservative ( or as an antioxidant) and
cleaning agent in nature. It is commercially obtained by fermentation process of
glucose with the aid of the mold Aspergillus niger and can be obtained
synthetically from acetone or glycerol. It can be used as an sour taste enhancer
in foods and soft drinks. The three carboxy groups lose protons in solution;
resulting in the excellent pH control as a buffer in acidic solutions. It is
used as a flavouring, stabilizing agent and acidulant (to control acidity) in
food industry, in metal-cleaning compositions as it chelates metals. Citric acid
is available in forms of anhydrous primarily and in monohydrate, the
crystallized form from water. The hydrated form will be converted to the
anhydrous form above 74 C. Citrate is a salt or ester of citric acid. Citrates
are formed by replacing the acidic one, two, or all three of the carboxylic
hydrogens in citric acid by metals or organic radicals to produce an extensive
series of salts, esters, and mixed (double) salts. Cirrates are used in food,
cosmetics, pharmaceutical and medicine industries as well as in plastic
industry; nutrient or food additives having functions of acidity regulator,
sequestering and stabilizing agent, antioxidants synergist, firming agent;
anticoagulant for stored whole blood and red cells and also for blood specimens
as citrates chelate metal ions and saline cathartics, effervescent medicines;
high boiling solvent, plasticizer and resin for food contact plastics. |
|
GENERAL DESCRIPTION OF BUFFER |
Buffer is a
substance, generally a solution, that can keep its pH constant, despite the
addition of strong acids or strong bases and external influences of temperature,
pressure, volume, redox potential. Buffer prevents change in the concentration
of another chemical substance, e.g., proton donor and acceptor systems that
prevent marked changes in hydrogen ion concentration (pH). Many acid-base
reactions take place in living organisms. However, for organisms to perform
certain vital functions, the body fluids associated with these functions must
maintain a constant pH. For example, blood must maintain a pH of close to 7.4 in
order to carry oxygen from the lungs to cells; blood is therefore a powerful
buffer. The commonest buffer in chemical solution systems is the acid-base
buffer.
·
Bicarbonate
buffer; a buffer system composed of bicarbonate ions and dissolved carbon
dioxide; in the body, this system is an important factor in determining the pH
of the blood as the concentration of bicarbonate ions is regulated by the
kidneys and of carbon dioxide by the respiratory system.
·
Cacodylate
buffer; one containing an organic arsenical salt, used in preparing fixatives
for electron microscopy.
·
Phosphate
buffer, a buffer system composed of KH2PO4 and Na2HPO4; in the body, it is
important in regulating the pH of the renal tubular fluids; when 0.025 molal
(equimolal of the potassium and sodium salts), the pH is 6.865 at 25
C.
·
Protein
buffer, a buffer system involving proton donor and proton acceptor groups of
the amino acid residues of proteins.
·
TRIS buffer
(tromethamine): an amine base used intravenously as an alkalizer for the
correction of metabolic acidosis. The pH values of all buffers are temperature-
and concentration-dependent. For Tris buffers, pH increases about 0.03 unit per
C temperature decrease, and decreases 0.03-0.05 unit per ten-fold
dilution.
·
Veronal
buffer; a barbital buffer commonly used in the preparation of fixatives for
electron microscopy.
|
